ClinicalTrials.gov
ClinicalTrials.gov is one of the worlds largest data bases of clinical trials. Registration to ClinicalTrials.gov is legally required for any drug or intervention seeking approval for use from the FDA. I was a UX designer on a multidisciplinary team undergoing a multi-year effort to modernize the public site and the back-end database.
The Challenge
The most pervasive challenges we addressed in redesigning the database included:
Vastly different user segments
Users include patients seeking trials to participate in, academic researchers, medical professionals
Dense technical information
There is a need to balance the level of detail required for medical professionals and scientists with understandability for those without knowledge of clinical research. The lack of plain language is a barrier to potential patients and their advocates.
Varying levels of data provided
Many data providers prefer to offer the least amount of information possible to comply with federal regulations for a variety of reasons ranging from wanting to protect their intellectual property to simply wanting to get registration done as quickly as possible. We need to find a way to show data providers the value in providing the thorough data on their studies.
Deliverables
Strategy
Impact maps
Research plan
Clinical trial lifecycle interactive system map
Research findings report
Coded user insights database
Clickable prototypes
Live website
Methods
Participatory workshops
User interviews
System data mapping
Key insights creation
Design Studios
Wireframing
User Testing
Problem Framing
Workshops
In order to better define the goals of this initiative, our UX team designed and facilitated a problem framing workshop in which we developed a strategy for the entire modernization effort. We started by imagining the future state of ClinicalTrials.gov and worked backwards taking into consideration the current challenges. 20 subject matter experts from the clinical trial community represented our different user groups.
Impact Map
Once we had the large scale vision of the modernization effort we had to get more specific on the actions needed to accomplish our goals. We represented each user group in the impact map to get a clearer picture of how we could achieve the desired outcomes. This tool was directly used to create our user stories and ensured that the entire team remained aligned as we moved forward in our modernization process.
Systems Thinking
Data Ecosystem Map
One thing we knew early on is that the lifecycle of a clinical trials spans far beyond it’s time on ClinicalTrials.gov. In order to fully understand the nuances of a clinical trial we create a Clinical Trial Data Ecosystem Map. This served as a visual to see all of the actors involved, the data included, and the broad milestones as a clinical trial moves from an idea to published study. The map is interactive, allowing for a broad overview and more detailed information on each element involved.
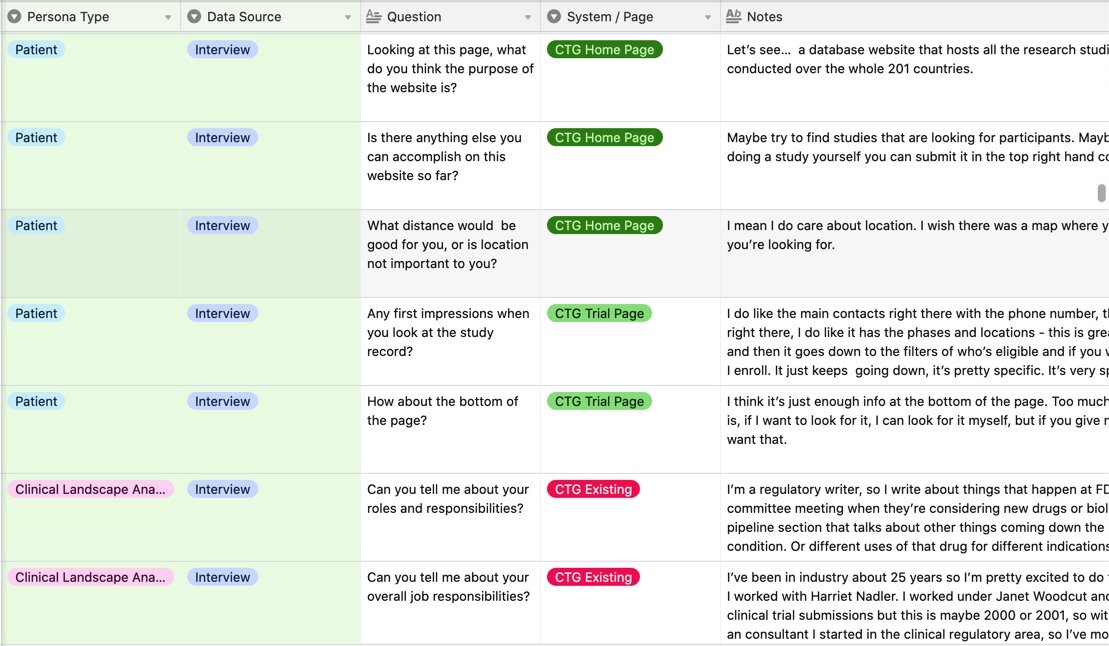
User Interviews
Coded Findings
We recruited patients and patient advocates, ensuring diversity amongst our participants and conducted interviews to get an understanding of
the process patients go through in order to find and enroll in studies
what information is most valuable to a patient when searching for a trial
what questions remain after finding a potential study
Personas
Through our user research we were able to create and validate 11 persona types that fell into 3 categories: Patients and their Advocates, Data Providers, and Data Consumers.
Ideation and Design
Design Studio Workshops
Our 10 person cross-functional agile team collaborated to create journey maps, and design critique sessions to educate non-designer partners about the benefits of designing with users. This allowed designing for and with users to become a full team effort at every stage of the design process. Once this base line understanding was achieved we led design studio workshops with our subject matter experts to solve for some of the challenges identified through our user research. Most notably we found through our user interviews that patients often thought that the studies listed on ClinicalTrials.gov are approved by the U.S. government. We posed the question to our design studio participants “how might we better communicate to patients that ClinicalTrials.gov is a library database of studies and that any study listed is not approved by the U.S. government?”
Prototypes
From our design thinking sessions we were able to create content in plain language form and revise the content architecture of the site in order to make it easier for the users to find and understand information. I then created an initial mockup of the homepage, search results page, and study details page using the United States Web Design System. As we continue with user testing we will continue iterating and improving the design.
User Testing
All of the mockups were initially tested on internal stakeholders for initial feedback. We then moved to 8 user testing sessions where the users were given tasks to navigate fully through the mockups. We also included highlighter testing with users to ensure they were able to understand the content. We are currently about to undergo another series of user testing on the newest iteration of the designs and content.
Future Considerations
When I left this project a solid foundation of understanding of our users and their needs had been established and the project was ongoing. While we were able to make immediate changes to the user interface and content language more work is needed across government agencies to resolve data opportunities in the larger ecosystem.